In addition to ISO 14971 there are several other key medical device industry standards requiring risk management. The ISO requirements for PDFA file viewers include color.

Iso 14971 Risk Management Plan Template
PDFA is an ISO-standardized version of the Portable Document Format PDF specialized for use in the archiving and long-term preservation of electronic documentsPDFA differs from PDF by prohibiting features unsuitable for long-term archiving such as font linking as opposed to font embedding and encryption.
. PDFX is formalized in ISO standards 15929 and 15930. ISO 14971 for m edical devices and two ge neral purpose. Organizations active in the medical.
ISO 134852016 Medical devices A practical guide has been authored by technical experts of ISOTC 210. Listen back to our free on-demand webinar. Dr Peter Bowness Medicinal and Biologics Technical Team Manager provides a summary of the updated ISO 14971 and the key.
Table B1 Correspondence between elements of ISO 149712007 and ISO 149712019 ISO 149712007 ISO 149712019 Introduction Introduction 1 Scope 1 Scope 21 accompanying document 31 accompanying documentation 32 22 harm 33 harm 34 hazard 35 hazardous situation 25 intended use 36 intended use intended purpose intended purpose 37 in. International Organizatio n for Stan dardization ISO has three standard s. ISO 15930 defines the specific implementations.
The template includes topics as required by clause 732 of ISO 134852016 and 82030b as required by 21 CFR 820. Add to cart SKU. The partial list includes.
The handbook is intended to guide organizations in the development implementation and maintenance of their quality management system in accordance with ISO 13485. And all these regulatory agencies endorse ISO 14971 Medical devices -- Application of Risk Management to Medical Devices. Design and development plan Free Template.
ISO 149712019 defines the international requirements of risk management systems for medical devices defining best practices throughout the entire lifecycle of a device. ISO 15929 which was withdrawn in March 2008 and no longer is an official standard specified the guidelines and principles for the development of PDFX standards.

Pdf Latest Risk Management Guideline Iso 14971 2019 Environmental Aspects Of Medical Device

Pdf Iso 14971 Medical Device Risk Management Standard

Medical Device Risk Management Iso 14971 Pdfcoffee Com

Medical Device Risk Management Iso 14971 Pdf Risk Management Hazard Analysis And Critical Control Points

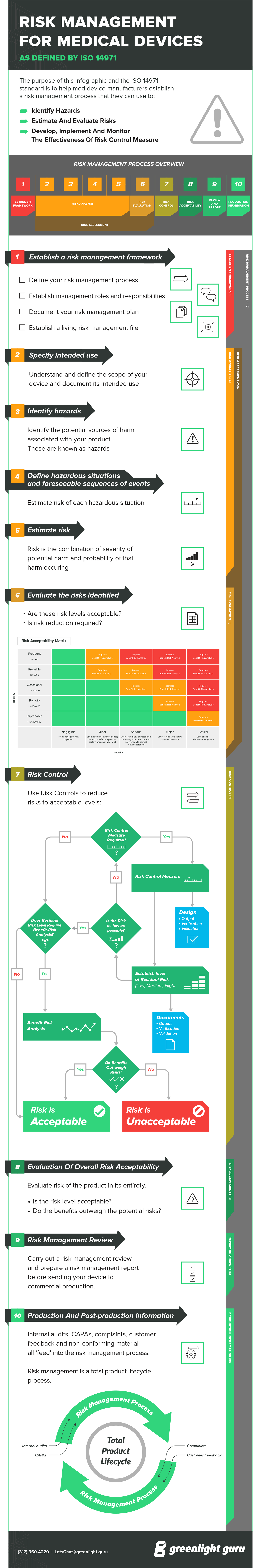
Iso 14971 Risk Management For Medical Devices The Definitive Guide

Iatf List Of Mandatory Documents Risk Analysis Documents List


0 comments
Post a Comment